Table of Contents
- A. Rationale for the New Deadlines for Proposal Submission
- B. Key Submission Steps and Deadlines
- C. Research and Administrative Contents
- D. Proposal Development with Pre-Award Services
- E. The Administrative Review Process at OSP
- F. Exceptional Circumstances
- G. Proposals Not Eligible for Late Submission Review
- Administrative Contacts
A. Rationale for the New Deadlines for Proposal Submission
This policy covers proposals for external funding, including grants, contracts, cooperative agreements, and Other Transaction Authority (OTA).
Federal agencies and other sponsors have significantly increased compliance requirements at the proposal stage, demanding more detailed documentation, certifications, restrictions, and training requirements. Moreover, the number of grant submissions has increased 18% since FY 2022. Given the increased competition for grant funding, this trend is likely to continue, adding further pressure on research administration staff. Establishing and enforcing firm internal deadlines will ensure adequate time for thorough compliance review, proper support to faculty, and enhanced proposal quality in an increasingly competitive and regulated funding landscape.
B. Key Submission Steps and Deadlines
B1. Overview of Key Steps and Deadlines
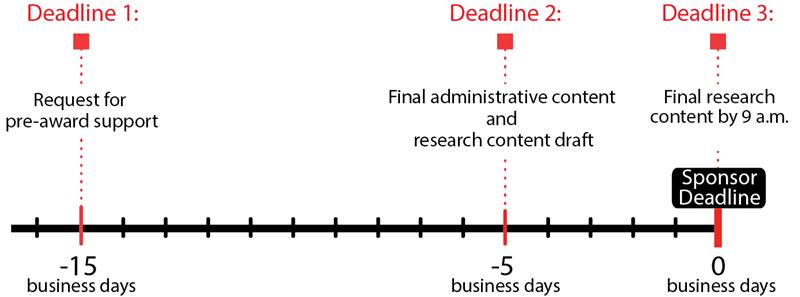
- Step 1. Request pre-award support for full OSP review: Send a request for pre-award services at least fifteen (15) business days before the sponsor deadline.
- Step 2. Submit content for full OSP review: Submit final administrative content and a research content draft to Office of Sponsored Programs (OSP), CALS Office of Preaward Services (OPAS), or CVM College Research Office (CRO) at least five (5) business days before the sponsor deadline.
- Step 3. Submit final research content: Submit final research content to OSP by 9 a.m. on the day of the sponsor deadline.
B2. Submission Steps and Deadlines
Note: Best practices are to submit requested materials as soon as you can ahead of indicated deadlines.
Step 1. Request pre-award support for full OSP review
Principal Investigators should submit a request for pre-award support as soon as they decide to write a proposal, but no later than fifteen (15) business days before the sponsor deadline. The request for pre-award support should be submitted to OSP/Pre-award Operations (PRO), CALS/OPAS, or the relevant department college/unit research administrator (see Section D for pre-award service information).
What to submit: OSP/PRO Proposal Notification form, CALS/OPAS Proposal Submission Form, or CVM Proposal Notification Form.
Note: Units that do not use OSP/PRO or CALS/OPAS services will have different processes for proposal notification. Please contact your department college/unit research administrator for any questions.
- Proposals including subrecipients: Subrecipient documents will be requested three (3) business days following receipt of proposal notification or no later than twelve (12) business days before the sponsor deadline.
Step 2. Submit content for full OSP review
Submit final administrative content and a research content draft to your proposal development specialist in OSP, CALS/OPAS, or CVM College Research Office (CRO). To allow time for a full administrative review, PIs must submit their content via pre-award services at least five (5) business days before sponsor deadline (by 9:00 a.m.).
Note: PIs must follow proposal support staff guidance and deadlines for submitting to OSP.
More information about administrative content, research content, and the review process is listed in Sections C and E below.
- What to submit: Final administrative content of proposal and draft of research content.
- Proposals including subrecipients: Subrecipients are required to submit their documents seven (7) business days prior to the sponsor deadline.
Step 3. Submit final research content
By no later than 9 a.m. on the day of sponsor deadline, PIs must submit final research content to their proposal development specialist at OSP/PRO, CALS/OPAS, CVM CRO, or unit research administrator. On the day of submission to the sponsor, the PI must be available to respond to follow-up questions in a timely manner.
- What to submit: Final research content of proposal.
Note: PIs should not expect detailed review of research content on the day of submission. For full review, final research content must be submitted five (5) business days before the sponsor deadline.
B3. Late Submission with Limited OSP Review:
For proposals that do not meet any of the criteria listed under Section G: Proposals Not Eligible for Late Submission below, PIs have the following late submission/ limited review options:
Step 1. Late Submission/Limited Review Option—Request for pre-award support
PIs that miss the initial 15-day deadline may still submit the proposal notification under the following conditions:
- When: At least ten (10) business days before the sponsor's deadline (by 9:00 a.m.).
What to submit: OSP/PRO Proposal Notification form, CALS/OPAS Proposal Submission Form, or CVM Proposal Notification Form.
Note: Units that do not use OSP/PRO or CALS/OPAS services will have different processes for proposal notification. Please contact your department college/unit research administrator for any questions.
- Review type: Limited administrative review only (see Section E).
- Eligibility: Only proposals that do not meet any of the criteria listed under Section G: Proposals Not Eligible for Late Submission below.
- Cut-off date: Proposals for which the proposal notification is received less than ten (10) days in advance of the sponsor deadline will not be submitted.
Step 2. Late Submission/Limited Review Option—OSP Review
PIs that miss the 5-day deadline may still submit the proposal to OSP, CALS/OPAS, or CVM CRO under the following conditions:
- When: At least three (3) business days before the sponsor's deadline (by 9:00 a.m.).
- What to submit: Complete final administrative content and a draft of research content.
- Review type: Limited administrative review only (see Section E).
- Eligibility: Only proposals that do not meet any of the criteria listed under Section G: Proposals Not Eligible for Late Submission below.
- Review cut-off date: If the required administrative materials are received less than three (3) days before the sponsor deadline, proposals will not be submitted to the sponsor.
Proceed to Step 3. Submit final research content, which has no late submission option.
B4. Additional Considerations
- This policy applies to all externally funded research and other sponsored activity proposals, including, but not limited to, federal, state, foundation, non-profit, and corporate funding opportunities. Proposals without a stated deadline also are subject to the timing requirements noted above.
- Awards received for proposals submitted without prior OSP review and approval will not be accepted by Cornell, unless they receive special approval by Dean/Director and Vice Provost for Research.
- Exemptions not subject to this policy include:
- Pre-proposals and letters of intent
- White Papers
- Proposal updates and Just-In-Time (JIT) requests
C. Research and Administrative Contents
C1. Research Content Includes (section labels may vary by sponsor):
- Project Summary/Abstract
- Narrative/Research Plan/Project Description
- Specific Aims
- References Cited
C2. Administrative Content Includes (depending on sponsor/proposal requirements):
- RASS (Research Administration Support System) record completed
- Sponsor application forms completed
- Senior/Key Personnel Documents
- CV(s)/Biosketch(es)
- Collaborators and Other Affiliations (COA) Information
- Current & Pending/Other Support
- Budget & Budget Justification
- Cost share commitments if applicable; these should not be included in the research content
- Appendices/Supplemental Documents including:
- Facilities, Equipment, and Other Resources
- Postdoctoral Mentoring Plan
- Resource Plan
- Data Management Plan
- Vertebrate Animals
- Human Subjects
- Subaward RASS record completed and subrecipient documents uploaded (letter of commitment, scope of work, budget, budget justification, and any other sponsor required documents)
- Letters of Support
D. Proposal Development with Pre-Award Services
Pre-award services for proposal development are provided by OSP’s Pre-award Operations (PRO) and CALS Office of Pre-award Services (OPAS) office, as well as by research administrators in some departments, colleges and units. These administrators assist with developing budgets, completing forms, compiling current and pending reports, and collecting biographical sketches.
Proposal development staff require time to prepare budgets, assemble application materials, and respond to the research teams’ questions and requests prior to OSP submission. PIs and proposal development staff need to work collaboratively to ensure that proposals are prepared in a timely manner to meet the proposal review deadline.
The following is a list of selected specific duties performed by pre-award services:
- Review and interpret sponsor solicitations for proposals
- Prepare summaries of required proposal documents and templates of common proposal elements
- Draft Current & Pending/Other Support reports
- Develop budgets, including detailed financial budget and narrative justification
- Prepare and assemble proposal packages, including completion of electronic applications
- Interface with internal and external collaborators to collect required documents including CV's, letters of support, subaward documents, etc.
- Initiate RASS project record and route for approval and review of Grant & Contract Officer (GCO)
- Liaise and collaborate with the assigned Grant & Contract Officer (GCO) through the successful submission of the application for funding
E. The Administrative Review Process at OSP
Pre-award services release the completed proposal to Grant and Contract Officers (GCOs) for administrative review. GCOs in OSP, CALS OPAS and CVM Research Office are the Authorized Organizational Representatives (AORs) responsible for proposal review and submission at Cornell. All proposals for sponsored funds must be reviewed and submitted via one of these offices, regardless of sponsor requirements.
E1. Sponsored proposals require thorough institutional review to ensure:
- Compliance with sponsor guidelines and evolving regulations
- Accuracy of budget calculations and institutional commitments, financial and non-financial
- Approval from department/unit chairs/directors, deans, and institutional officials
- Coordination with other institutional offices including compliance, export control, finance, and international affairs
E2. Full administrative review includes, but is not limited to:
- Compliance with sponsor guidelines/request for proposals
- Accuracy of proposal record in RASS
- Completion of PI/co-PI attestations and unit approvals
- Compliance with PI eligibility requirements
- Compliance with federal and state requirements
- Accuracy of budget and budget justification
- Inclusion of appropriate indirect cost and benefit rates
- Comparison of other support/current & pending support documents against Cornell records
- Compliance of biosketches/CVs with sponsor guidelines
- Appendices/supplemental documents
- Subaward documents, if applicable
- Completion of institutional certifications
- Compliance with Cornell policies and requirements
E3. Limited administrative review includes:
- Institutional compliance (certifications, approvals, inclusion of appropriate indirect cost rate, cost share approval, additional required resources and commitments).
F. Exceptional Circumstances
Proposals may be considered after the internal deadline in the following exceptional circumstances without requiring the escalation procedure:
- Late announced opportunities: direct sponsor deadline announced less than ten (10) business days prior to deadline.
- Invitation-only-submissions for which formal invitations were issued with short notice.
F1. Escalation procedure for emergencies and other extreme cases
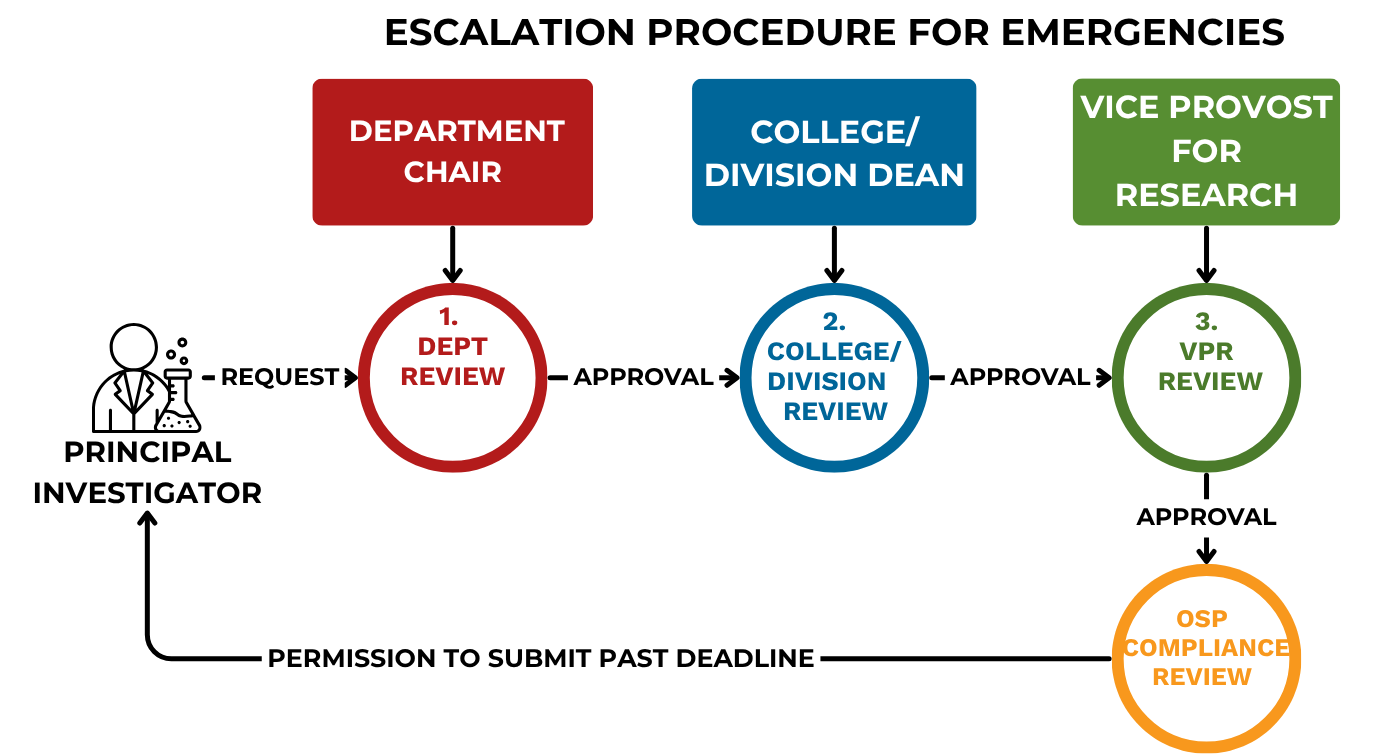
To request approval for submitting a proposal after the internal deadline, please follow this process:
- Department Review–The PI must email the Department Chair (or equivalent) to request review and submission of the proposal. The email should explain the reason for the delay and why the proposal could not meet the 5-day full review or 3-day limited review deadline.
- College/Division Review–If the Chair recommends approval, the request will be forwarded to the Dean of the College/Division who will analyze the request.
Vice Provost for Research (VPR) Review–If the College/Division recommends approval, the request will be forwarded to the Vice Provost for Research (VPR), who has final authority to grant an exception.
OSP Compliance Review–When a request for late submission has been approved by Chair, Dean, and VPR, OSP will review the proposal only for institutional compliance (certifications, approvals, inclusion of appropriate indirect cost rate, cost share approval, additional resources and commitments required). OSP will notify the PI they have permission to submit past the deadline.
G. Proposals Not Eligible for Late Submission Review
The proposal is not eligible for late submission review if any of the following apply:
Cost share included
Total budget exceeds $10,000,000
Subawards included
Response to RFPs that are exemptions from Cornell policies, including but not limited to export control, publication restrictions, IP ownership, citizenship restrictions, sponsor anonymity, and similar restrictions
Response to contract or Other Transaction Authority (OTA) solicitations
Foreign sponsors, including foreign universities
Proposals requiring space commitments, e.g., changes to laboratory/office space, additional space, any renovation
Administrative Contacts
OSP continually strives to provide exemplary service to researchers. Feedback on OSP’s review process, or any other aspect of our services, is encouraged and should be sent to osp-help@cornell.edu.
GCOs have in-depth knowledge and expertise with specific sponsors and can work with researchers and departmental representatives or units to guide them through the proposal process. Please consult OSP at any time with questions or feedback directly or using the following contact information:


